Research
Establishing niche-specific macrophage fates
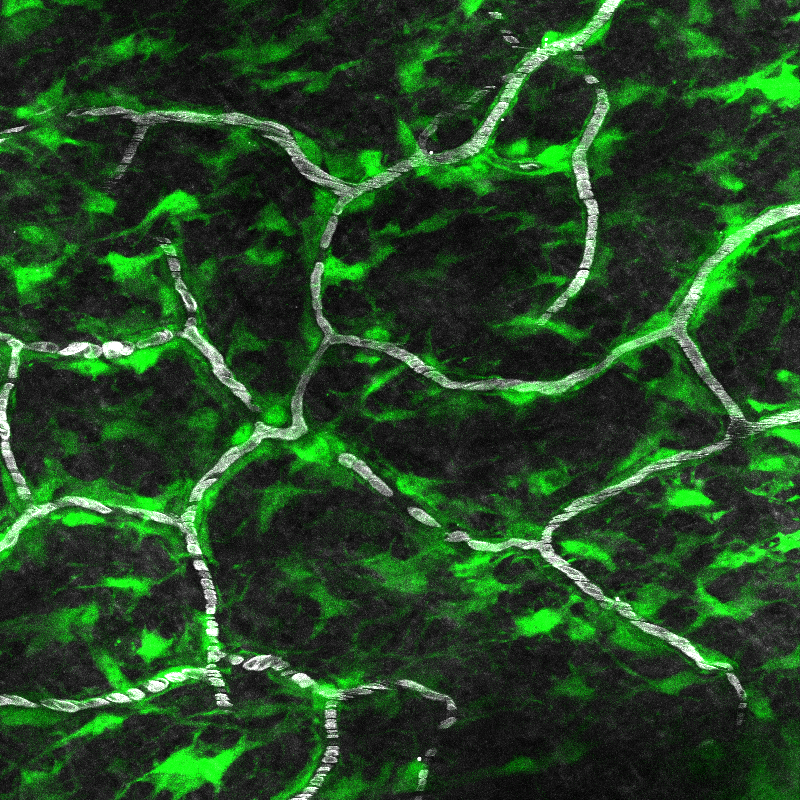
Resident immune cells remain localized to distinct tissue niches and adopt unique functions to support local homeostasis. Selective loss of these niche-specific cells leads to inflammation and tissue dysfunction. Despite this fundamental insight, it remains unclear how these cells selectively establish a resident fate to contribute to local tissue health. Utilizing multiphoton intravital microscopy in the mouse skin, the lab aims to identify the sequence of cellular behaviors and molecular cues that drive immune cell recruitment and integration into a tissue niche.
Wound-induced immune cell dynamics
Following tissue injury and immune cell recruitment, a tissue can either (1) form a scar or (2) regenerate the lost tissue structures. In mammalian skin, immune cells (including macrophages) can drive both scarring and regenerative outcomes. Therefore, the lab aims to identify the early tissue dynamics and molecular signals of immune cells that yield a permissive environment for regenerative outcomes, which can be leveraged to overcome current limitations in tissue regeneration.
Local immune cell aging and tissue dysfunction
Aged tissues contain dysfunctional immune cells that contribute to impaired wound healing and pathogen clearance. However, it remains unclear the source of these age-associated immunological defects. Do aged tissues drive immune cell dysfunction? Or do aged immune cells locally corrupt a tissue over time? To address these fundamental questions, I propose to leverage novel spatial transcriptomic approaches based on in vivo niche-specific fluorescent labeling in mammalian skin to unify immune cell transcription, location, and behavior as tissues progressively age.